How Can We Help?
High Risk Biologicals Import Application – Human Cell Lines
For shipping high risk biological products, such as human cell lines, virus, etc. Please contact us for further guidance and information.
Guide to Customs Affairs (000129013000)
Guidance on Permit Application for “Examination and Entry of Special Items for Sanitary Quarantine”
This application may be applied by the end user of the biological products to be imported.
Online application website: http://tswp.customs.gov.cn
Basic Information
| Application Type | Administrative Permit | Office Type | Commitment |
| Implementation Office | Responsible for the customs health departments | Execution Level | National/Provincial |
| Chargeable | No | Application forms | Apply in person or apply online |
| Number of office visits | 1 | Explanation of the number of office visits | 0 for apply online 1 for apply in person |
| Commitment time limit | 20 working days | Statutory closing time | 20 working days |
| Description of commitment time limit | The decision on whether to grant permission shall be made within 20 working days from the date of acceptance. Expert data review, on-site assessment, laboratory testing, etc. are not included in the statutory closing time limit, but must be carried out as soon as possible. In principle, the review of expert data should not exceed 20 working days, and the on-site assessment within the country should not exceed 2 months. The laboratory test time depends on the test items and methods, and should not exceed 2 months. |
| Legal time limit for settlement | 20 working days from the date of issue of the Acceptance Decision. |
| Consulting method | Customs consultation telephone number (link to each customs website) or 12360 customs service hotline |
| Monitoring complaint methods | Customs consultation telephone number (link to each customs website) or 12360 customs service hotline |
| Processing time | (1) On-site application. The working hours of the on-site examination and approval department of each directly-affiliated customs (link to each of the directly-affiliated customs websites) or dial 12360 customs service hotline. (2) Apply online. Business application time: 24 hours. Customs review time: Monday to Friday, the working hours of each customs or subordinate customs. |
| Location | Apply online: The applicant logs in the “Internet + Customs” integrated online service platform (website: http://online.customs.gov.cn), enters the “Sanitary Quarantine” module, selects the “Special Articles Sanitary Quarantine” function, and completes the declaration online as required Single or go to the relevant business window of the customs directly under the site. |
Supporting Materials
| Name | Template | Type | Source | Copies | Required |
| Application Form for Import-Export Special Articles Sanitary Quarantine | Click to Download | Original | Applicant | 1 electronic copy | Yes |
| Descriptive materials for entry and exit special items (Chinese and English names, categories, ingredients, sources, uses, special sales channels, import / export countries or regions, manufacturers, etc. of special items) | N/A | Copy/Scan | Prepared by Applicant | 1 electronic copy | Yes |
| Import Registration Certificate issued by the Drug Supervision and Administration Department of the State Council (biological products and human blood products used for the prevention, diagnosis, and treatment of human diseases when entering the country shall be provided) | Click to Download | Original | Government issued | 1 electronic copy | Yes |
| Chinese and English names of the pathogenic microorganisms and their biological characteristics in English and Chinese (except for entry or exit special articles that contain or may contain pathogenic microorganisms) | N/A | Copy/Scan | Prepared by Applicant | 1 electronic copy | Yes |
| Proof documents that the production operator or user has the corresponding level of biosafety prevention and control (Special articles for entry and exit that contain or may contain pathogenic microorganisms should be provided) | Click to Download | Original | Prepared by Applicant | 1 electronic copy | Yes |
| Sales certificate issued by the drug supervision and administration department (biology products and human blood products that are used for the prevention, diagnosis, and treatment of human diseases shall be provided when leaving the country) | Click to Download | Original | Government issued | 1 electronic copy | Yes |
| Approval document issued by the human genetic resources management department (the special items for exit involving human genetic resources management should be provided) | N/A | Original | Government issued | 1 electronic copy | Yes |
| The biosafety laboratory qualification certificate that is compatible with the biosafety risk level, laboratories with BSL level 3 or higher must be accredited by a national accreditation body (Units using special entry-exit items that contain or may contain pathogenic microorganisms should provide) | N/A | Original | Government issued | 1 electronic copy | Yes |
| Approval document from the competent health department of the people’s government at or above the provincial level (the entry or exit of highly pathogenic pathogenic microorganisms (toxic) species or samples should be provided) | N/A | Original | Government issued | 1 electronic copy | Yes |
| Risk assessment report | N/A | Original | Prepared by Applicant | 1 electronic copy | Yes |
| Description of the basic situation of the unit (management system certification, unit address, production site, laboratory settings, storage facilities and equipment, product processing, production process or process, floor plan, etc.) | N/A | Copy/Scan | Prepared by Applicant | 1 electronic copy | Yes |
| ID card | N/A | Copy/Scan | Government issued | 1 electronic copy | Yes |
Detail Description
I. Name of the matter: Approval of sanitary quarantine for entry-exit special items
Type of matter: Administrative Permit
Setting basis:
(I) “Implementation Rules of the Frontier Health and Quarantine Law of the People’s Republic of China”
Article 11 The carriers, shippers, or postal carriers of special articles such as microorganisms, human tissues, biological products, blood, and their products that enter or leave the country must declare to the health and quarantine office and accept the health quarantine. Customs clearance procedures for special items. No entry or exit is allowed without the permission of the health and quarantine authorities.
(2) “Administrative Provisions on Sanitary Quarantine for Entry-Exit Special Articles” (Order No. 160 of the former General Administration)
Article 6 The customs directly under the jurisdiction shall be responsible for the examination and approval of sanitary quarantine (hereinafter referred to as the examination and approval of special articles) for special articles in and out of the jurisdiction.
Implementing agency: responsible for the health department of the customs
V. Statutory closing time: 20 working days from the issuance of the Acceptance Decision.
6. Commitment to settle the time limit: a decision on whether to grant permission is made within 20 working days from the date of acceptance. Expert data review, on-site assessment, laboratory testing, etc. are not included in the statutory closing time limit, but must be carried out as soon as possible. In principle, the review of expert data should not exceed 20 working days, and the on-site assessment within the country should not exceed 2 months.
7. Name of the result: “Entry / Exit Special Item Sanitary Quarantine Examination and Approval Form”
Sample results: see Annex 1
Nine, Charge Standard: No Charge
10. Basis of Charge: None
Eleven, application conditions:
(1) Where laws and regulations require the approval documents of relevant departments, the corresponding approval documents shall be obtained;
(2) Having the ability to control biosafety in accordance with special items for entry and exit.
Application Materials:
Fill in the application materials online, no need to submit paper application materials.
(1) When applying for approval of special articles, the owner or his agent shall provide corresponding materials in accordance with the following provisions:
1. Application Form for Sanitary Inspection and Quarantine of Special Items for Entry / Exit (Annex 2, 3);
2. Descriptive materials for entry and exit special articles, including Chinese and English names, categories, ingredients, sources, uses, major sales channels, import / export countries or regions, manufacturers, etc. of special articles;
3. For entry of biological products and human blood products used for the prevention, diagnosis and treatment of human diseases, an import registration certificate (Annex 4 and 5) issued by the drug regulatory department of the State Council shall be provided;
4. For entry or exit special articles that contain or may contain pathogenic microorganisms, the scientific names (Chinese and Latin) of the pathogenic microorganisms, explanatory documents of biological characteristics (Chinese and English), and the producers or operators or users have the corresponding Proof of biosafety prevention and control level (Annex 6);
5. Biological products and human blood products used for the prevention, diagnosis and treatment of human diseases exiting the country shall provide a sales certificate issued by the drug regulatory department (Annex 7);
6. If the exit special items involve the scope of human genetic resources management, the approval document issued by the human genetic resources management department shall be provided (Annex 8);
7. Units that use entry-exit special items that contain or may contain pathogenic microorganisms should provide biosafety laboratory qualification certificates that are compatible with the level of biosafety risk (Annex 9). Laboratories above BSL-3 must obtain national accreditation Approval of
8. For entry or exit of highly pathogenic pathogenic microorganisms (toxic) species or samples, the approval document of the competent health department of the people’s government at or above the provincial level shall be provided (Annex 10);
9. When entering the risk assessment process, a risk assessment report must be provided.
(2) If the applicant is an entity, in the first application for approval of special items, in addition to the materials specified in item (1) of these guidelines, basic information about the entity, such as the certification of the organization’s management system, the address of the organization, the production site, Laboratory settings, storage facilities and equipment, product processing, production processes or technological processes, floor plans, etc.
If the applicant is a natural person, a copy of the identity card shall be provided. Applicants for entry and exit of pathogenic microorganisms or special articles that may contain pathogenic microorganisms shall not be natural persons.
XIII. Application procedures:
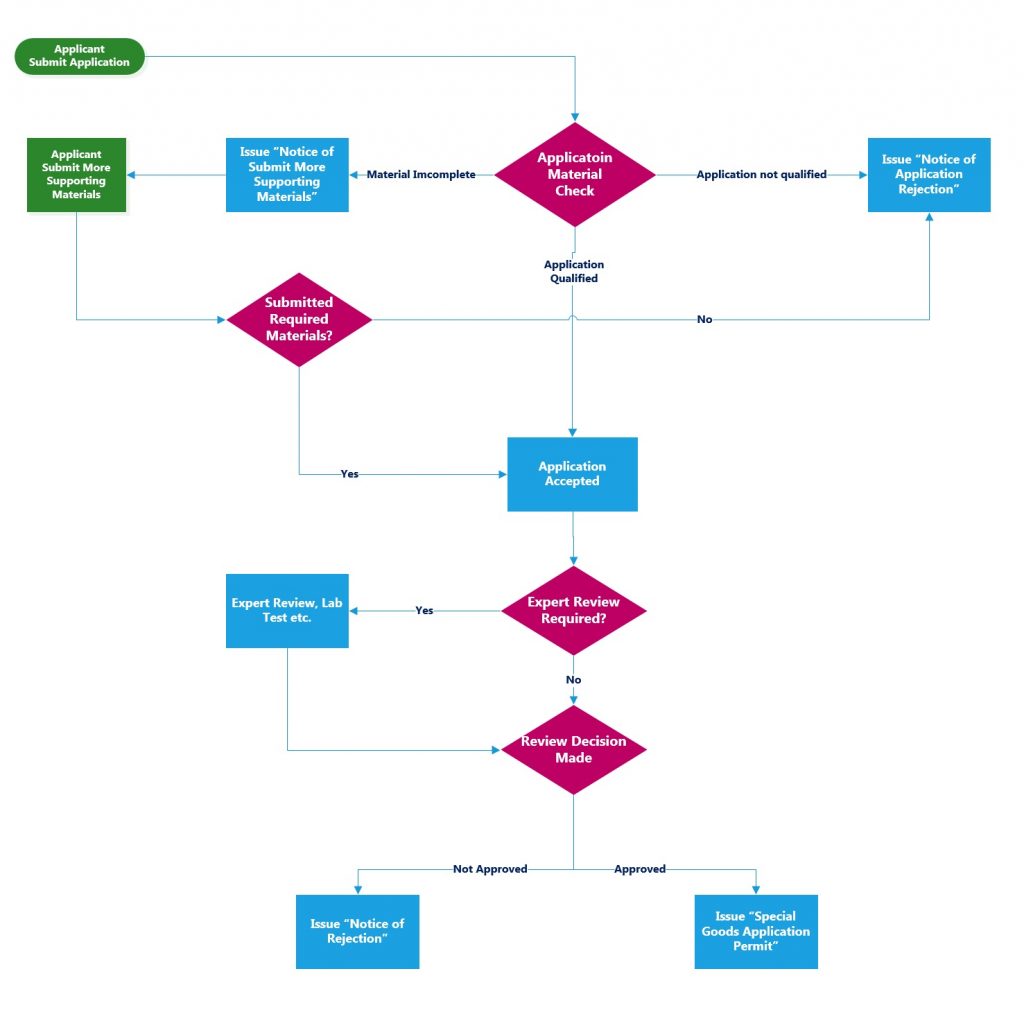
Application (online, all day) Acceptance (If the applicant finds that the application is wrong and needs to be corrected, it can be withdrawn by the customs before acceptance.) Preliminary review (organize risk assessment if necessary) Review and decision (20 working days from the date of acceptance, no (Including time for risk assessment and laboratory testing).
14. Form of application:
Apply online or on-site.
15. Number of visits to the scene:
0 online or 1 on-site.
16. Examination criteria:
(1) Whether the content of the application form is truthful and accurate.
(2) Whether the uploaded documents are true and clear (certificates, certifications, filing and approval documents, etc. must be scanned copies of the original or stamped and endorsed with a “copy checked with the original” copy).
(3) Whether the uploaded documents meet the requirements (if laws and regulations require the approval of relevant departments, the corresponding approval documents shall be obtained).
(4) Whether the relevant units have the ability to control biosafety in accordance with the special items for entry and exit.
17. Scope of General Affairs: Customs Customs Areas
18. Make an appointment: No (you can submit an application at any time)
19. Online payment: No (not involved)
20. Logistics Express: No (you can check the approval result online at any time and print it yourself).
21. Location:
Apply online: Applicants log in to the “Internet + Customs” integrated online service platform (website: http://online.customs.gov.cn), enter the “Sanitary Quarantine” module, select the “Special Articles Sanitary Quarantine” function, and complete the declaration online as required Single or go to the relevant business window of the customs directly under the site.
22. Processing time:
(1) On-site application. The working hours of the on-site examination and approval department of each directly-affiliated customs (link to each of the directly-affiliated customs websites) or dial 12360 customs service hotline
(2) Apply online. Business application time: 24 hours.
Customs review time: Monday to Friday, the working hours of each customs or subordinate customs.
Twenty-three, consultation telephone: the consultation telephone of each subordinate customs (link to each subordinate customs website) or 12360 customs service hotline.
24. Supervisory telephone: consultation telephone of each customs office (link to each customs website) or 12360 customs service hotline.
